NK cells are innate lymphoid cells critical for mediating anti-tumor responses and are being advanced as a cancer immunotherapy. While conventional (c) NK cell-based therapies have been explored in clinical trials, challenges related to cancer target recognition, in vivo persistence, and functionality result in limited activity. To overcome these challenges, NK cell memory programs have been investigated. Cytokine-induced memory-like (CIML or ML) NK cells are generated after brief activation with IL-12, IL-15, and IL-18, resulting in enhanced functionality, cytotoxicity, and in vivo persistence. ML NK cells have been advanced in multiple clinical trials for leukemia with promising results. Despite this translational progress, the underlying cellular dynamics and molecular mechanisms of ML reprogramming remain unclear. Elucidating these molecular programs may optimize NK therapeutics and reveal new translational strategies. We hypothesized that transcriptional and epigenetic mechanisms contribute to ML NK cell heterogeneity arising after IL-12/15/18 activation.
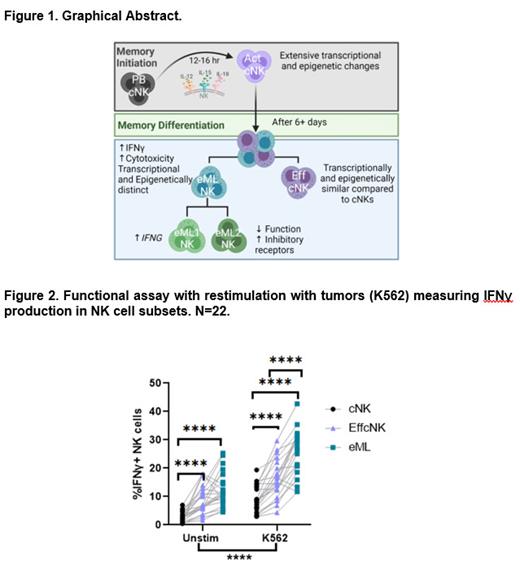
To test this, NK cells from healthy donors were activated with IL-12/15/18 (to become ML) or IL-15 (to maintain cNK) and differentiated in vitro for 7 days (Fig 1, n=3-40 donors), followed by the assessment of their transcriptome (CITE-seq) and epigenome (ATAC-seq). CITE-seq clustering revealed unexpected heterogeneity with ~50% clustering with cNK cells (effector, effcNK) and ~50% forming a unique enriched ML cluster (eML). eML clusters were significantly increased in frequency in the IL-12/15/18 condition, compared to cNK (Mean±SEM=53±10.5% for IL-12/15/18, 6.2±1.1% for IL-15, p<0.0001) and discovered to expressed ENTPD1 (CD39). Based on CITE-seq, a flow cytometry strategy was developed to distinguish eML NK cells from effector cNK and cNK cells. Next, we identified eML NK cells 3-4 weeks after adoptive transfer of IL-12/15/18 activated donor NK cells across three different clinical trials (mean=13.3±1.9%, p<0.001). eML NK cells produced significantly more IFNg compared to cNK cells and effector cNK cells when stimulated with leukemia targets (Fig. 2, p<0.0001), and had enhanced cytotoxicity compared to cNK cells (EC50 of eML=0.862, EC50 of cNK=2.223, p<0.0001). Bulk ATAC-seq on flow-sorted populations revealed that eML NK cells have a distinct global epigenetic profile with >4,500 significant differentially accessible regions (DARs) compared to effcNK, while effcNK and cNK cells were epigenetically similar. Further, we interrogated which transcription factors are enriched in DARs of eML NK cells revealing TBOX and IRF family motifs. Within CITE-seq, the eML cluster was comprised of two subsets (eML-1 and eML-2). eML1 had increased expression of the transcription factors TCF7 and TOX2. In contrast, eML-2 had increased expression of IRF4 and PRDM1, as well as terminal exhaustion genes, including LAG3 and TIGIT (p<0.05). To evaluate eML-1 and eML-2 function, we performed CITE-seq after stimulation of eML NK cells with leukemia targets. eML-1 NK cells were the most functional, with significantly increased IFNG compared to eML-2, while eML-2 was hypofunctional and marked by high TOX expression (p<0.05). These findings suggest that optimizing strategies that lead to eML-1 differentiation may enhance the therapeutic activity of IL-12/15/18-induced ML NK cells.
In summary, we identify heterogeneity following IL-12/15/18 activation of NK cells, resulting in multiple cellular fates. Further, these data identify an eML NK cell population with a distinct single-cell transcriptional and epigenetic program that directly manifests as enhanced NK cell functional response to leukemia.
Disclosures
Foltz:Nationwide Children's Hospital: Patents & Royalties: WO 2019/152387, US 63/018,108 (licensed to Kiadis); EMD Millipore: Other: monoclonal antibody unrelated to the present work licensed to EMD Millipore; CPRIT: Consultancy. Becker-Hapak:Washington University in St Louis: Patents & Royalties: US20100159594A1. Cubitt:Pionyr Immunotherapeutics: Divested equity in a private or publicly-traded company in the past 24 months. Berrien-Elliott:WUGEN: Consultancy, Current holder of stock options in a privately-held company, Patents & Royalties. Cashen:Kite Pharma: Consultancy. Bednarski:Horizon Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sobi: Membership on an entity's Board of Directors or advisory committees. Fehniger:Affimed: Other: Scientific Advisory Board; Indapta: Current holder of stock options in a privately-held company; Orca Bio: Current holder of stock options in a privately-held company; HCW Biologics: Research Funding; Smart Immune: Other: Scientific Advisory Board; AI Proteins: Other: Scientific Advisory Board, Research Funding; Wugen: Consultancy, Current holder of stock options in a privately-held company, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal